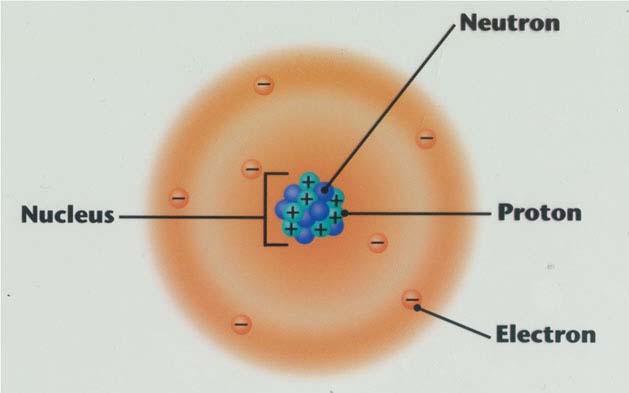
The smallest piece of matter that can still have properties of its element is called an atom. Atoms are too small to be seen by the human eye, so of course there are theories about how atoms look and work.
- One of the first atomic theories that surfaced was proposed by a physicist named John Dalton. John Dalton was born in the mid 1700's and died in the early 1800's. Dalton started teaching at the age of 12, and later became a teacher of mathematics and natural philosophy at a local college.
- Dalton believed that all elements were made of tiny particles called atoms, and that all atoms of a single element were identical. Dalton also claimed that all atoms of one element were different from the atoms of any other element. He said that atoms of one element could combine with atoms of another element and form chemical compounds. Dalton proposed that atoms could not be created, divided nto smaller particles, or destroyed in chemical processes; The chemical reaction only changed the way the atoms were arranged in the elements. The model that Dalton made looked similar to a billiard ball because it was only a round, solid sphere.
 |  |
- J.J. Thomson was one of the next physicists to make an improvement to the atomic model. While working with gas discharge tubes, Thomson discovered that there was more to an atom than just a solid sphere. J.J. Thomson discovered rays in the tube that moved from the negative end of the tube to the positive end of the tube. Thomson theorized that the rays were made of negatively charged particles called electrons.
- After more experimenting with atoms and the rays of electrons, Thomson proposed that all atoms contained small, negatively charged particles called electrons that were embedded evenly throughout the positively charged sphere. His model was called "the plum pudding model," though some of us today might call it "the chocolate chip cookie dough model" because the electrons were like the chocolate chips in the cookie dough.
 |  |
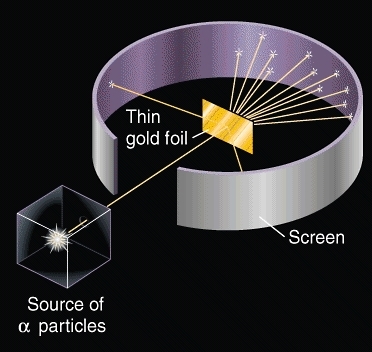
- The next physicist that made a major breakthrough with the atomic theory was Ernest Rutherford. Rutherford conducted an experiment with a sheet of gold foil. In the experiment, Rutherford shot rays of particles at the sheet of gold foil and observed what happened. In his observations, Rutherford noticed that not all the rays went straight through the foil, but some bounced off, or went through the foil and came out at a different angle. With these observations, Rutherford concluded that the atom was mostly empty space, but consisted of a positively charged nucleus and negatively charged electrons that orbited around the nucleus.
- While Rutherford was working, he had another physicist named Niels Bohr learning from him. After Rutherford proposed his new model of the atom, Niels Bohr started working to improve it. Bohr concentrated his work on the electrons in the atom. Like Rutherford, Bohr did believe that the electrons orbited around the nucleus, but he thought there was more to it than just electrons moving freely around it. Bohr proposed the idea of "electron energy levels," fixed paths that electrons that carried different amounts of energy travelled on. These energy levels kept the electrons from being attracted to the postively charged nucleus. Bohr also proposed that when electrons moved from energy level to energy level, that they gave off light, and to move up an energy level, the electron would gain energy, but to move down, they would lose energy.
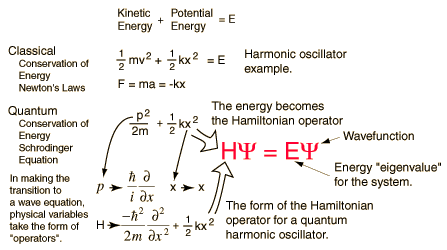
- The next physicist who wanted to improve the atomic theory was Werner Heisenberg. At first, Heisenberg agreed with the Bohr model of the atom, but quickly determined that if you couldn't see what you were proposing, then it was most likely impossible. So instead of being on determined paths that were unable to be seen, Heisenberg proposed that the momentum and position of the particles in an atom would have to be calculated by complex mathematical equations.
- Erwin Schrodinger developed the equation that is still used to understand atoms today. This equation is called the Schrodinger equation, unfortunately, it is difficult to solve even for the simplest of atoms.
The current accepted model of the atom is the "Electron Cloud Model" which was a compound of all the formerly proposed atomic theories.